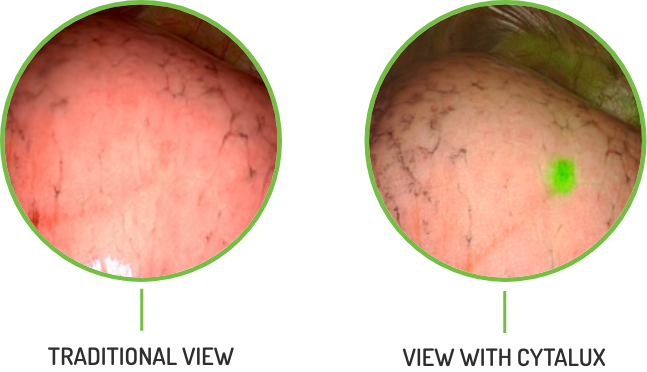
See Cancer In Real Time
CYTALUX® (pafolacianine) injection is the only targeted molecular imaging agent that illuminates ovarian and lung cancer intraoperatively.
CYTALUX in Action
See how CYTALUX enhances surgeons’ ability to visualize lung and ovarian lesions.
See CYTALUX in actionCYTALUX is indicated as an adjunct for intraoperative identification of malignant lesions in adult patients with ovarian cancer and malignant and non-malignant pulmonary lesions in adult patients with known or suspected cancer in the lung.
In clinical trials, CYTALUX detected additional ovarian and lung cancer
that wouldn’t have been detected otherwise.
Ovarian Cancer Trial
In 27% of patients, additional lesions were found that were not identified with conventional methods.
Lung Cancer Trial
In 19% of patients, the primary lung lesion was localized, but not detected with conventional methods.
In 8% of patients, one or more synchronous malignant lesions not detected by pre-operative imaging was identified.
* Women highly suspicious for or with confirmed ovarian cancer who underwent both normal and fluorescent light evaluation (Intent-to-Image set); N=134, 95% CI [19.6, 35.2]


Speak with a representative
about CYTALUX and how to bring this technology to your OR.