Efficacy and Safety
Phase 3 (006 Study):
CYTALUX® FOR FR+ OVARIAN CANCER
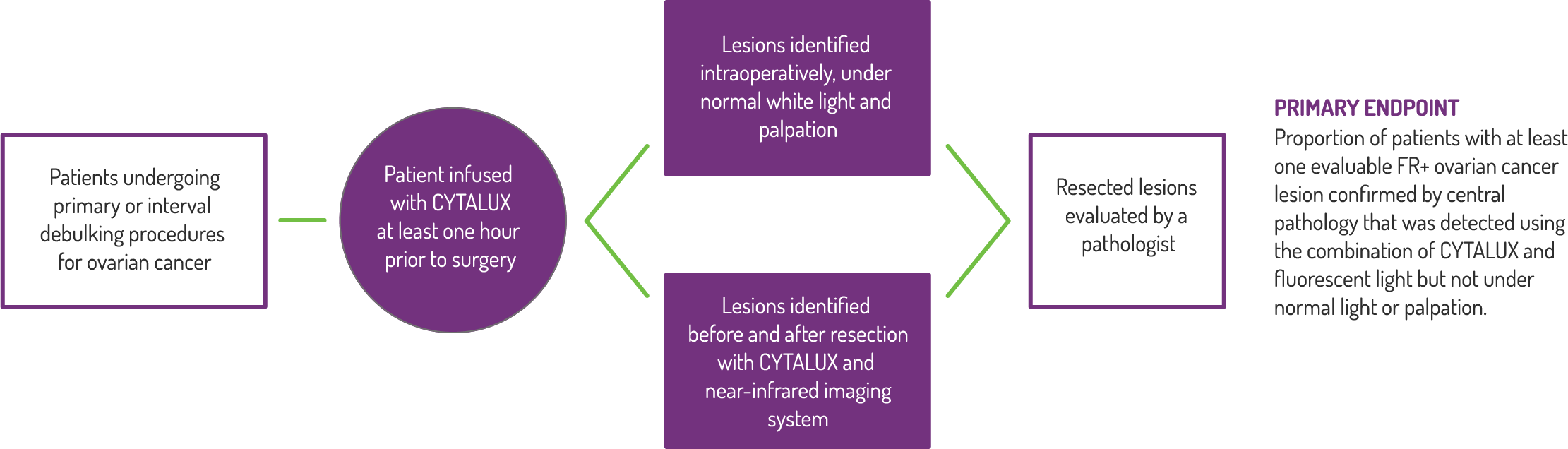
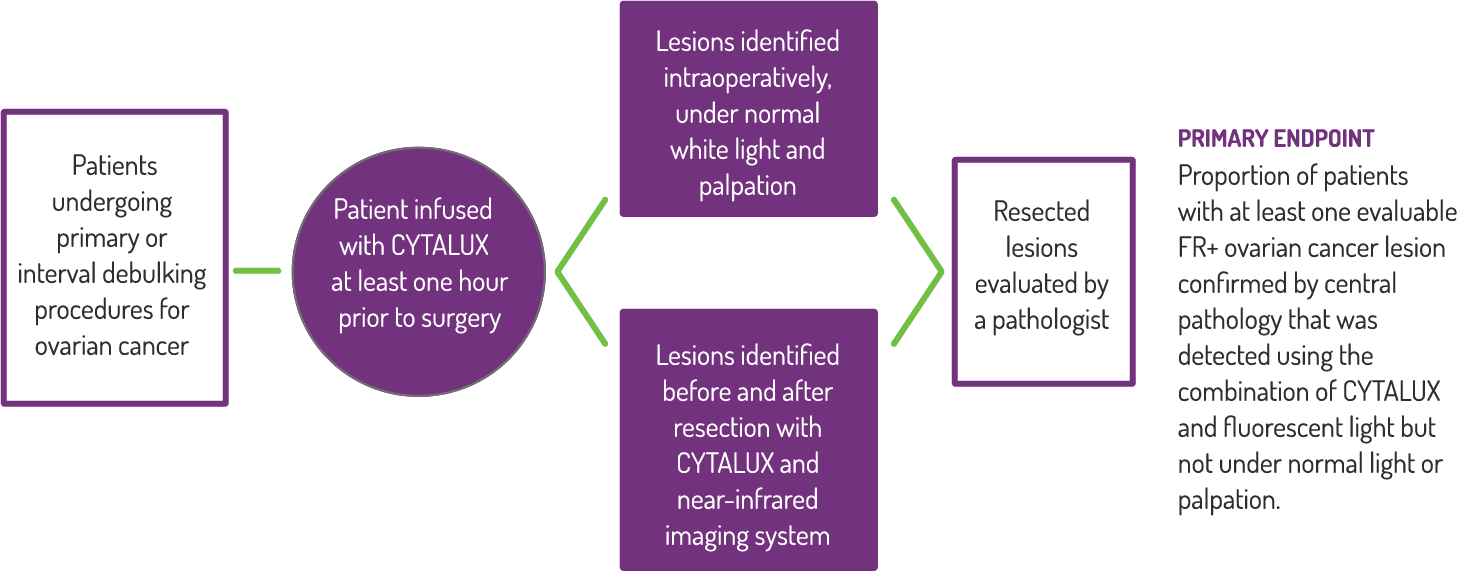
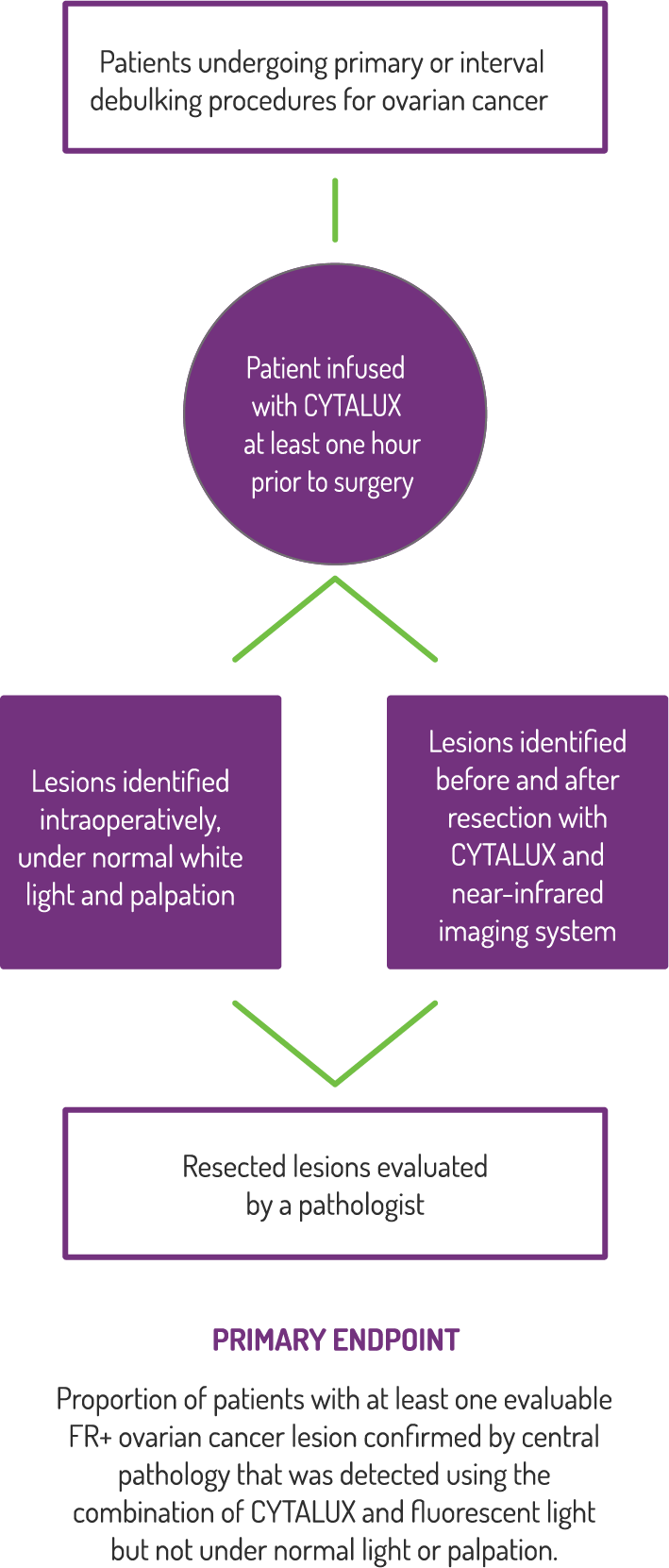
Study Design
A Phase 3, randomized, single dose, open-label study to investigate the safety and efficacy of CYTALUX for intraoperative imaging of folate receptor positive ovarian cancer.
Efficacy
With CYTALUX, additional lesions were found in 27% of patients.*
In a subgroup analysis of patients with confirmed FR+ ovarian cancer who underwent interval debulking surgery, additional lesions were found in 40% of patients.†
Surgeons indicated the use of CYTALUX altered their surgical plan in 54% of their cases.‡
* Women highly suspicious for or with confirmed ovarian cancer who underwent both normal and fluorescent light evaluation (Intent-to-Image set); N=134, 95% CI [19.6, 35.2]
† Phase 3 (006 Study): CYTALUX FOR FR+ OVARIAN CANCER; N=58, 95% CI [27.0, 53.4]
This subgroup analysis utilized a smaller analysis set than the primary endpoint and was not adjusted to control for error, so the results are not conclusive and should be interpreted cautiously.
‡ Phase 3 (006 Study): Based on a prespecified exploratory endpoint for the proportion of subjects for whom the fluorescence surgical plan was changed based on fluorescence imaging both prior to initiation of the surgical procedure and upon re-imaging of the surgical field after surgical procedure prior to closing. (N=109, 95% CI [44.3,63.7])
Drug-Related Adverse Events
| DRUG-RELATED ADVERSE EVENTS | Mild/Moderate n (% n/N) | SEVERE n (% n/N) |
|---|---|---|
| Subjects with at least one drug related TEAE | 43 (28.7%) | 2 (1.3%) |
| Total number of drug- related adverse events | 63 | 2 |
drug-related serious adverse events were observed in any patient dosed with CYTALUX.
| MOST COMMON DRUG-RELATED ADVERSE EVENTS | MILD/MODERATE n (% n/N) | SEVERE n (% n/N) |
|---|---|---|
| Nausea | 27 (18.0%) | 0 |
| Vomiting | 8 (5.3%) | 0 |
| Abdominal Pain | 7 (4.7%) | 0 |
of all drug-related adverse events were mild-to-moderate.
drug-related serious adverse events were observed in any patient dosed with CYTALUX.
of all drug-related adverse events were mild-to-moderate.
N=150 study subjects infused with CYTALUX
Misinterpretation of Camera Images


Speak with a representative
about CYTALUX and how to bring this technology to your OR.