Efficacy and Safety
ELUCIDATE (Trial 007): Enabling Lung Cancer Identification Using Folate Receptor Targeting
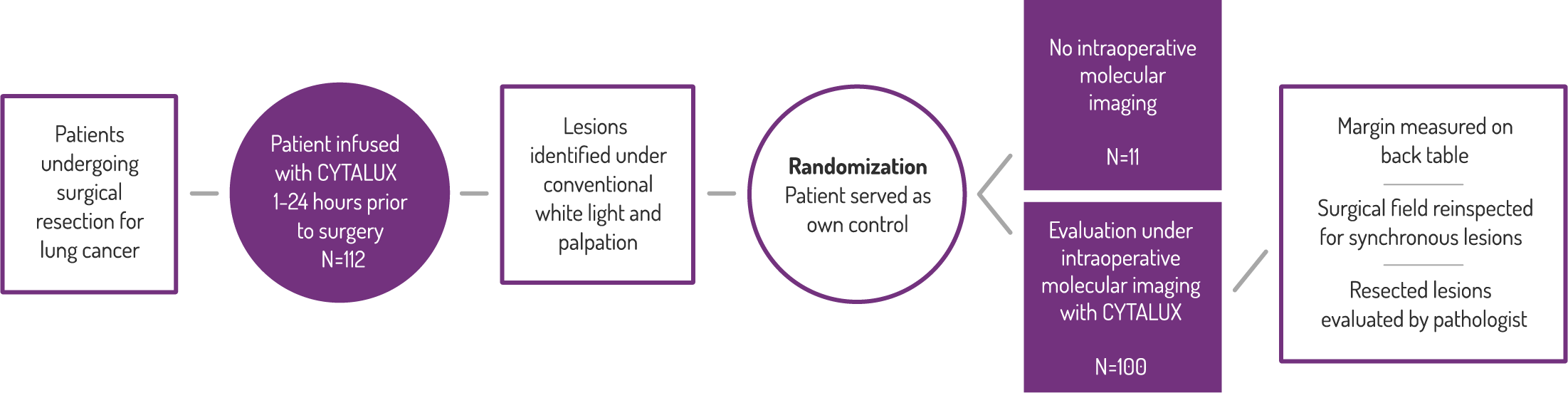
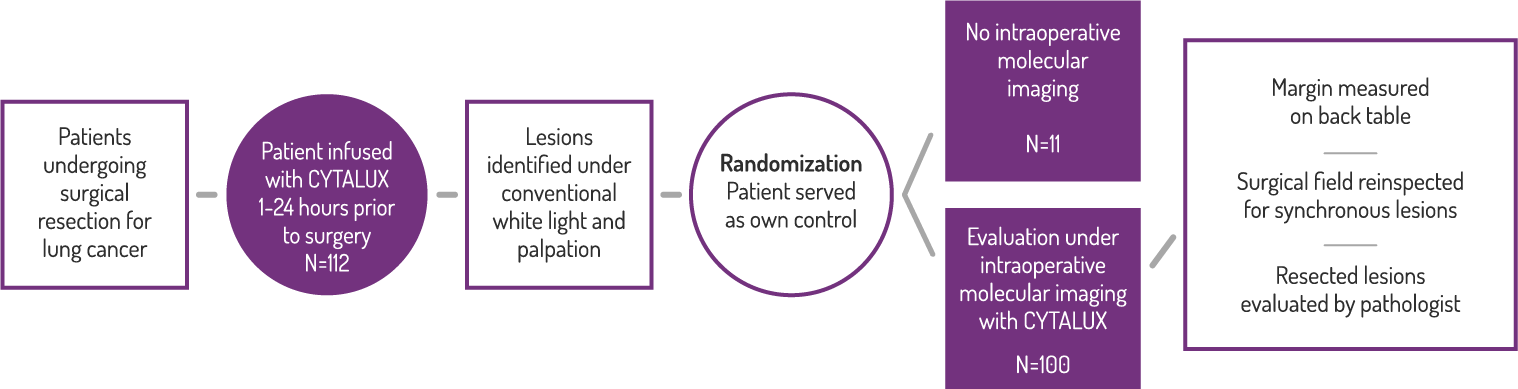
Study Design
A Phase 3, randomized, single dose, open-label trial that investigated the safety and efficacy of CYTALUX® for intraoperative imaging of folate receptor positive lung lesions.
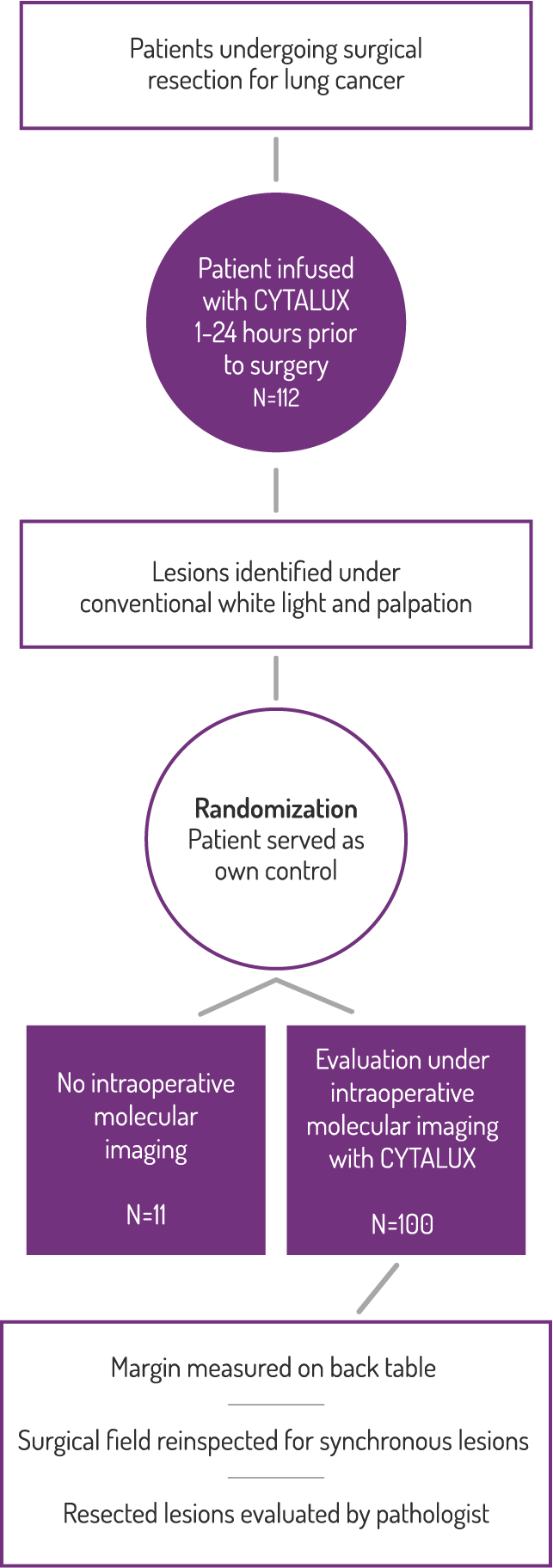
Primary Endpoint: Clinical Significant Events
Localization of
pulmonary lesion
Detection of one or more
synchronous malignant lesions
Identification of a close
resection margin
Efficacy
In 38% of patients, a close resection margin (≤10mm) was identified.*
Surgeons reported changing the scope
of their procedure in 29% of patients,
due to use of CYTALUX.†
* Sarkaria IS, Martin LW, Rice DC, Blackmon SH, Slade HB, Singhal S; ELUCIDATE Study Group. Pafolacianine for intraoperative molecular imaging of cancer in the lung: The ELUCIDATE trial. J Thorac Cardiovasc Surg. 2023 Mar 3:S0022-5223(23)00185-X. doi: 10.1016/j.jtcvs.2023.02.025. Epub 2023 Mar 02. PMID: 37019717.
† Based on a prespecified exploratory endpoint for the proportion of subjects for whom the surgical plan was changed based on fluorescence imaging both prior to initiation of the surgical procedure and upon re-imaging of the surgical field after surgical procedure prior to closing. (N=100, 95% CI [20.4, 38.9])
LESION SIZE
| Size of lesions identified by CYTALUX and not by standard white light in Phase 3 Trial* | MEDIAN | MINIMUM |
|---|---|---|
| Primary Lesions | 13 mm | 5 mm |
| Synchronous lesions | 12 mm | 2 mm |
LESION depth
| Depth of lesions detected by CYTALUX in Phase 3 Trial* | MEDIAN | MAXIMUM |
|---|---|---|
| Detected by CYTALUX only | 10.1 mm | 27.9 mm |
| Detected by CYTALUX and white light | 2.3 mm | 37.7 mm |
Drug-Related Adverse Events
ELUCIDATE Phase 3 Trial
| DRUG-RELATED ADVERSE EVENTS | MILD/MODERATE n (%) | SEVERE n (%) |
|---|---|---|
| Subjects with at least one drug-related TEAE✝ | 36 (32.1%) | 3 (2.7%) |
| Total number of drug-related TEAEs✝ | 55 | 5 |
drug-related serious adverse events were observed in any patient dosed with CYTALUX.
| MOST COMMON (>2%) DRUG- RELATED ADVERSE EVENTS | MILD/MODERATE n (%) | SEVERE n (%) |
|---|---|---|
| Nausea | 10 (8.9%) | 0 |
| Vomiting | 4 (3.6%) | 0 |
| Intermittent hypertension | 3 (2.7%) | 1 (0.9%) |
of all drug-related adverse events were mild-to-moderate.
drug-related serious adverse events were observed in any patient dosed with CYTALUX.
of all drug-related adverse events were mild-to-moderate.
✝TEAE = Treatment Emergent Adverse Event
N=112 study subjects infused with CYTALUX
Misinterpretation of Camera Images


Speak with a representative
about CYTALUX and how to bring this technology to your OR.